Abstract
Introduction FMS-like tyrosine kinase 3 (FLT3)-internal tandem duplication (ITD) and -tyrosine kinase domain (TKD) mutation were common mutations in acute myeloid leukemia (AML). FLT3-ITD mutation adversely affected the outcome of hematopoietic stem-cell transplantation (SCT). While sorafenib and midostaurin as maintenance therapy after SCT prolonged relapse-free and overall survival (OS), there are very few reports of post-transplant gilteritinib maintenance therapy outside of clinical trials.
Hence, to explore the efficacy of the gilteritinib maintenance therapy post-SCT, we here report the prognostic impact of post-SCT gilteritinib maintenance in a clinical setting.
Methods We retrospectively analyzed 25 FLT3-mutated relapsed or refractory AML patients who received allogeneic SCT at our center from January 1, 2011 to April 30, 2022. FLT3 mutation was analyzed by a commonly used polymerase chain reaction (PCR)-based assay. Measurable residual disease (MRD) was assessed by Wilms tumor 1 (WT1) mRNA expression in peripheral blood, and MRD positivity was defined as WT-1 >50 copies/μg in this study. Relapse was defined as an increase of 5% blasts or more. Non-relapse mortality (NRM) was defined as death without relapse. Leukemia-free survival (LFS) was defined as the time from transplantation to leukemia relapse or death from any cause, which comes first. OS was defined as the time from transplantation to death from any cause.
Results The median age was 52 years. Twenty-two patients had ITD mutation and 5 had TKD mutation (2 had both ITD and TKD mutation). At the time of the transplant, 20 patients were in remission (6 were MRD-negative and 14 were MRD-positive) and five were in non-remission. All patients achieved hematologic complete remission (CR) after SCT and no patients relapsed within 30 days post-SCT. After hematological engraftment, 14 patients started to receive gilteritinib as maintenance therapy and 11 patients did not. Between the two groups, the patients' characteristics including disease status at transplant, SCT procedures, and donor sources were well balanced except for the administration of FLT3-inhibitor prior to SCT (100% in patients with gilteritinib maintenance and 45.5% in those without gilteritinib maintenance, p = 0.003). Eleven patients (78.6%) in the gilteritinib group and eight (72.7%) patients in the non-gilteritinib group had residual MRD in hematological CR or were non-CR prior to SCT. The median time from SCT to the initiation of gilteritinib was 36 days. The median dose at gilteritinib initiation was 40mg (range 20-120 mg). One patient developed grade 2 skin acute graft-versus-host disease (GVHD) after gilteritinib administration and 4/14 patients (28.6%) developed moderate-severe chronic GVHD (cGVHD, two developed pulmonary cGVHD, one eye and mouse, and one skin) with a median onset of 10.5 months (range 3.1-30.5 months), while only one patient temporary discontinued gilteritinib until the improvement of cGVHD. Late engraftment failure during gilteritinib maintenance was not observed.
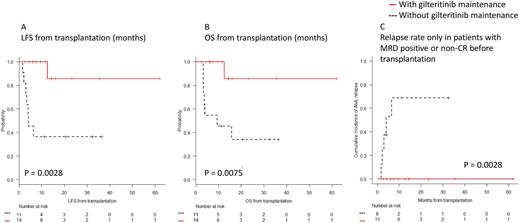
In terms of survival benefit, patients with gilteritinib maintenance showed significantly longer LFS and OS than those without gilteritinib (1-y LFS; 100% vs. 36.4%, 95% CI; 100-100% vs. 11.2-62.7%, p = 0.0028, Figure A, and 1-y OS; 100% vs. 45.5%, 95% CI; 100-100% vs. 16.7-70.7%, p = 0.0075, Figure B). The cumulative incidence of AML relapse at one-year in patients with gilteritinib maintenance group was significantly lower than those without (0% vs. 51.5%, 95% CI; 0-0% vs. 24.9-86.0%, p = 0.005). Especially, among patients with MRD positive or non-CR prior to SCT (n=19), patients who received gilteritinib maintenance showed a lower cumulative incidence of AML relapse at one-year than those without gilteritinib maintenance (0% vs. 68.8%, 95% CI; 0-0% vs. 35.9-95.2%, p = 0.0028, Figure C). Patients without MRD prior to SCT did not relapse with or without gilteritinib maintenance. These results suggest that gilteritinib maintenance therapy contributes to preventing post-transplant relapse in patients who remain MRD positive at the time of SCT.
Conclusion Gilteritinib maintenance therapy post-SCT showed survival benefit in a clinical setting.
Disclosures
Asada:Nippon Shinyaku: Speakers Bureau; Meiji: Speakers Bureau; Kyowa KIRIN: Speakers Bureau; Astellas: Speakers Bureau; Asahi KASEI: Speakers Bureau; Abbvie: Speakers Bureau; Novartis: Research Funding, Speakers Bureau. Ennishi:Eisai Pharmaceutical Co., Ltd.,: Honoraria; Chugai Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Kyowa Kirin Pharmaceutical Co., Ltd.: Honoraria; Nipponshinyaku Pharmaceutical Co., Ltd.,: Research Funding. Maeda:Eisai: Honoraria; Kyowa Kirin: Honoraria; Takeda: Honoraria; AstraZeneca: Research Funding; Astellas: Honoraria, Research Funding; Chugai Pharmaceutical: Honoraria, Research Funding; Nippon Shinyaku: Honoraria, Research Funding; Novartis Pharma: Research Funding, Speakers Bureau; Otsuka Pharmaceutical: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal